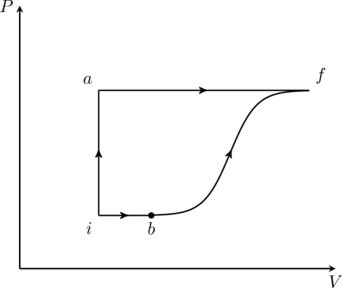
JEE Advanced 2014 Paper 1, Question 20
A thermodynamic system is taken from an initial state ![]() with internal energy
with internal energy ![]() to the final state
to the final state ![]() along two different paths
along two different paths ![]() and
and ![]() , as schematically shown in the figure. The work done by the system along the paths
, as schematically shown in the figure. The work done by the system along the paths ![]() and
and ![]() are
are ![]() and
and ![]() respectively. The heat supplied to the system along the path
respectively. The heat supplied to the system along the path ![]() and
and ![]() are
are ![]() and
and ![]() respectively. If the internal energy of the system in the state
respectively. If the internal energy of the system in the state ![]() is
is ![]() and
and ![]() , the ratio
, the ratio ![]() is
is
Solution
The problem gives us partial information about internal energies, heat transferred, and work done at various points in the PV diagram. We can complete the picture by using the first law of thermodynamics (see note)
(1) ![]()
where ![]() is the work done by the system. We apply this first to the path
is the work done by the system. We apply this first to the path ![]() . Note that no work is done along
. Note that no work is done along ![]() since volume is constant, therefore
since volume is constant, therefore ![]() . However, the internal energy at
. However, the internal energy at ![]() can be different from that at
can be different from that at ![]() (for example,
(for example, ![]() for an ideal gas undergoing an isochoric/constant-volume process, which means its internal energy
for an ideal gas undergoing an isochoric/constant-volume process, which means its internal energy ![]() changes). Thus,
changes). Thus,
(2) ![]()
Similarly, along the path ![]() ,
,
(3) ![]()
Finally, along ![]()
(4) ![]()
Therefore, the ratio ![]() .
.